Stanford Blood Center Implements Individual Donor Assessment for Blood Donation
As of Thursday, October 19, Stanford Blood Center (SBC) has implemented the updated FDA blood donation guidelines, which eliminate questions based on sexual orientation. We look forward to welcoming those who may be newly eligible to support local patients through blood donation.
Stanford Blood Center is committed to achieving an inclusive blood donation process that treats all potential donors equally and ensures a safe, sufficient blood supply is readily available for patients in need. This historic change in approach to donor eligibility is significant progress, resulting in a blood donation process that is more inclusive and equitable than ever before. Stanford Blood Center enthusiastically supports this change, aligning the United States with countries like Canada and the United Kingdom.
About the Individual Donor Assessment (IDA)
The new process focuses on assessing all donors equally, regardless of gender, reflecting a data-driven approach to maintaining blood safety. This ensures fairness and recognizes that infectious diseases can affect anyone. Ultimately, a thorough donor history questionnaire and extensive testing remain in place to ensure the safety of our blood supply.
The Changes
Previously, a man who had sex with another man within the last three months was deferred for three months following their last sexual encounter. Additionally, a woman was deferred in the past three months if she had sex with a man who had sex with another man in the past three months. Individuals were assessed based on the gender they identified with, and nonbinary individuals were evaluated using both criteria.
Under the new guidance, the FDA recommends an “individual donor risk assessment” approach that does not depend on gender or sexual orientation, and all donors will be asked the same questions about high-risk sexual behavior. More specifically, any donor who reports having a new partner or more than one partner in the past three months will be asked a follow-up question about anal sex. If anal sex with a new partner or multiple partners is reported in the past three months, the donor will be deferred for three months following the sexual encounter.
The new guidance also requires a three-month deferral for anyone who has taken an oral PrEP (pre-exposure prophylaxis) or PEP (post-exposure prophylaxis) medication to prevent HIV transmission. A two-year deferral is required if an injectable, long-acting PrEP or PEP medication is taken. A permanent deferral remains for anyone with a history of HIV infection.
Stanford Blood Center’s Role In the Changes
For many years, SBC has taken a leadership role in helping the FDA gather the required data to make this change by participating as an enrollment site for the ADVANCE Study (results from this study are partly responsible for the FDA’s shift in guidance), working with AABB on changes to the Universal Donor History Questionnaire (UDHQ), and directly advocating for prioritization of this change with members of congress as part of America’s Blood Centers. This work has demonstrated that this new eligibility screening process ensures a safe blood supply, and all patients may continue to trust that the blood they receive is safe.
Stanford Blood Center remains committed to achieving further progress and will continue to provide data to the FDA to make blood donation even more inclusive. We recognize the hurt the previous FDA policy that restricted gay and bisexual men from blood donation has caused. These are just the first steps in repairing relationships with the broader LGBTQ+ community. Stanford Blood Center welcomes everyone who wishes to be part of our humanitarian mission.
Resources:
The Individual Donor Assessment at a Glance
Blood Donor Eligibility
All blood donors must meet FDA eligibility criteria to donate blood every time. These eligibility criteria apply to all U.S. blood collection organizations. The FDA requires all potential blood donors to answer questions related to their health history before each donation. The donation criteria and questions on the health history questionnaire are designed to ensure that the blood collection process is as safe as possible for the donors and recipients of blood. Learn more about blood donation eligibility.
Health History Screening
During the pre-donation health history screening, Stanford Blood Center uses a questionnaire developed by the blood industry’s professional organization, AABB, and approved by the FDA to assess an individual’s health history. Health history questions are based on past and current behavior risks (for example, travel, medication, sexual activity, etc.) Sexual activity questions are based on specific behaviors, not on sexual orientation. The words “have sexual contact with” and “sex” are used in some of the questions and apply to any sexual activities (vaginal, oral, or anal), regardless of whether a condom was used during the activity.
Who Is Impacted
Under the FDA’s donor assessment eligibility criteria, the donor history questionnaire is gender-neutral, and all donors will answer the same questions regardless of gender or sexual orientation. This includes sexual behavior questions to assess individual risk factors. Any individual, irrespective of gender or sexual orientation, who has had new or multiple sexual partners in the last three months and had anal sex in that timeframe, will be asked to wait three months to donate blood from the last anal sex contact.
HIV Preventative Medications (PrEP/PEP)
If you have taken a drug to prevent an HIV infection, known as pre-exposure prophylaxis and post-exposure prophylaxis (PrEP or PEP), you are asked to wait three months from the last oral dose and two years from the last injection to donate blood. The waiting period is required due to these drugs interfering with viral replication and thus possibly altering the detectability of diagnostic and screening tests for HIV, including extending the window period before detectable infection or a delay in producing antibodies.
FAQs
In May 2023, the U.S. Food and Drug Administration (FDA) published final guidance on recommendations for evaluating donor eligibility using individual health history assessments to reduce the risk of HIV transmission by blood and blood products. The previous blanket deferrals for gay and bisexual men and women who have sex with them are eliminated.
The donor history questionnaire used to screen all prospective blood donors will now ask all donors a series of questions about their sexual history as a way of identifying donors with a potentially high risk for HIV. These questions include:
- Whether the individual has had multiple sexual partners in the past three months.
- Whether the individual has had any new sexual partners in the past three months.
If the answer is yes to either of the above questions, the individual will be asked about a history of anal sex in the past three months.
- If donors have had a new sexual partner or more than one sexual partner and had anal sex in the past three months, they will be deferred from donation for three months.
- Donors who have not had new or multiple sexual partners in the past three months will not be asked about anal sex and may be eligible to donate, provided all other eligibility criteria are met.
- Donors with new or multiple sexual partners without anal sex may be eligible to donate, provided all other eligibility criteria are met.
Based on these questions, a temporary deferral of prospective donors may occur.
The FDA’s final guidance proposes a shift to create equitable donor eligibility standards for blood donors by using the same sexual behavior-based questions in all pre-donation screenings. These proposed changes align with current policies in countries like the United Kingdom and Canada, which have made similar changes in recent years. The FDA evaluated and utilized data from ongoing surveillance of the U.S. blood supply and results from studies conducted by other countries. The FDA also sponsored a recent research, Assessing Donor Variability and New Concepts in Eligibility (ADVANCE), to evaluate data among domestic populations.
This guidance has been updated twice, first when the lifetime ban for gay and bisexual men was lifted in 2015 and then again when the 12-month deferral for gay and bisexual men was reduced to three months in 2020.
Under the FDA’s new donor assessment guidance, a subset of gay and bisexual men will be able to now donate blood. There will no longer be specific questions related to men who have sex with men. Any individual, regardless of gender or sexual orientation, who has had new or multiple sexual partners in the last three months and had anal sex in that timeframe, will be asked to wait three months to donate blood from the last anal sex contact. Potential donors, including gay and bisexual men, who have not had a new partner or multiple partners in the past three months will be eligible to donate blood.
Under the FDA’s donor assessment, the MSM policy has been eliminated. Individuals who have been deferred under the previous MSM policy in the past may be eligible to donate once the three-month wait period from their last MSM deferral has passed, providing they meet all other eligibility criteria.
If you have been deferred at Stanford Blood Center in the past for MSM, please reach out to the SBC Resource Nurse at 650-725-7336 to confirm your eligibility and begin the process of requalification prior to coming in to donate.
A new sexual partner includes someone you have sex with for the first time or someone you had sex with in the past and then stopped but had sex with that person again in the last three months.
Statistically, anal sex has a significantly higher chance of HIV transmission per sex act than vaginal or oral sex due to the potential for blood exchange. This does not account for individuals’ safe sex practices but is based on an evidence-based approach to overall risk. Additionally, condoms have a significantly higher failure rate in anal sex than other forms of sex.
According to the FDA, condom use, while an excellent sexual health practice, isn’t an evidence-based method of screening donors because condoms are ineffective and can break or slip. We also know from research that questions about condom use are less reliably answered because people don’t always recall accurately.
No. At SBC blood donors are able to provide the gender they identify with, and this is what is added to the donor record and used to assess criteria that are gender-specific (such as hemoglobin measurements).
There are no donor eligibility criteria related to being transgender. Donors can report the gender they identify at the time of donation. Stanford Blood Center staff members must verbally confirm demographic information, including gender, with all presenting donors. This step helps ensure donor safety and the accuracy of records. If Stanford Blood Center records have the incorrect gender, prospective donors may ask staff members to make the change upon registration. Individuals do not need to tell staff that they are transgender.
Individuals with specific questions about eligibility can contact us at 888-723-7831.
There are no donor eligibility criteria related to being intersex. Donors can report the gender they identify with at the time of donation. Individuals do not need to tell staff that they are intersex, and can choose from one of three genders: female, male, or nonbinary.
There are no donor eligibility criteria related to being nonbinary or gender non-conforming. Stanford Blood Center is one of the few blood centers in the country that allows donors to select a gender of nonbinary. Under the FDA’s donor assessment guidance, the donor history questionnaire is gender-neutral, and all donors will answer the same questions regardless of gender. However, some gender-specific eligibility criteria still exist — such as height-to-weight ratio for certain donation types and iron (hemoglobin) levels. For nonbinary donors, the more conservative criteria are used to ensure donor safety.
Individuals with specific questions about eligibility can contact us at 888-723-7831.
We recognize that some people may be in sexual relationships with multiple people where their partners are not new. Under the FDA’s donor assessment guidance, people with multiple sexual partners who have engaged in anal sex with one or more partners in the last three months must wait three months from the last anal sex contact to donate.
If you have taken a drug to prevent an HIV infection, known as pre-exposure prophylaxis and post-exposure prophylaxis (PrEP or PEP), you are asked to wait three months from the last oral dose and two years from the last injection to donate blood. The waiting period is required due to these drugs interfering with viral replication and thus possibly altering the detectability of diagnostic and screening tests for HIV, including extending the window period before detectable infection or a delay in producing antibodies. They may delay the detection of HIV by licensed screening tests for blood donations, potentially resulting in false negative results.
If you have ever taken a drug to treat an HIV infection, known as antiretroviral therapy or ART, you are deferred from blood donation permanently since antiretroviral drugs do not eliminate the virus from the body, and donated blood can potentially still transmit HIV infection to a transfusion recipient.
Stanford Blood Center and the FDA support individuals making responsible choices for their health and the broader health of our communities. Stanford Blood Center and FDA are involved in ongoing research, data collection, and assessment related to transfusion safety, including HIV preventative medications. They will continue seeking opportunities that could help lead to additional changes. Stanford Blood Center does not encourage individuals to stop taking these medications to donate blood.
Dr. Peter Marks of The U.S. Food and Drug Administration indicated in a January 27, 2023, press conference that the FDA will continue to monitor data for possible changes in the future.
Stanford Blood Center tests each unit of donated blood for several infectious diseases. While testing has greatly improved, it is not 100 percent effective at detecting infectious diseases in donors with very early infection. The FDA selected the 3-month deferral to provide adequate time for detecting infected individuals. Learn more about blood donation screening tests.
Although testing has improved, it is not 100% effective at detecting infectious agents at the time of donation from individuals with very early infection or those who may be on preventative treatment for HIV and have a break-through HIV infection. The three-month deferral was selected to provide adequate time for developing other infectious disease markers in individuals with early infection—not just HIV. Each infectious disease agent (including hepatitis B and C) has a different length of time following infection where tests may not detect the virus, referred to as the window period. While the average time for HIV may be 9-10 days, this isn’t true of all infected persons, especially if they are on preventative treatment for HIV. Additionally, the window period for hepatitis B, on average, may be a few weeks.
No. Having a safe and adequate blood supply from volunteer blood donors is in the best interests of all patients requiring blood transfusions. The blood community has united in support of this change and has advocated for change for many years due to the excellent safety record of current infectious disease tests and more recent data on the risks associated with the transmission of HIV. The previous time-based deferral alienated a subset of the population due to its focus on sexual orientation rather than individual sexual behavior. The new guidance proposes screening protocols that will be applied to all donors regardless of sexual orientation, assessing all donors equally for potential high-risk behavior.
No. Based on the data available to the U.S. Food and Drug Administration today, the decision was made with confidence that it would continue to support a safe and robust blood supply. The new screening protocols will apply a set of screening questions about sexual behavior to all donors vs. a small subset of donors, which supports a safety screening methodology.
We don’t know, but we hope so. Approximately 68% of adults in the U.S. are eligible to donate blood voluntarily, yet only about 3% donate each year. Donation rates among donors under 50 years of age continue to decline, and less than 20% of donations come from individuals from communities of color, leading to concerns about the long-term resiliency of the blood supply. A donor screening process that equitably applies to all donors may help support a robust blood supply. It is too early to tell how many donors will or will not be deferred with the new criteria.
Spanning over many years, SBC has taken a leadership role in helping the FDA gather the required data to make this change, including regularly providing de-identified data samples, participating as an enrollment site for the ADVANCE Study (which is partly responsible for the FDA’s change in guidance), working with AABB on changes to the UDHQ, and directly advocating for prioritization of this change with members of congress as part of America’s Blood Centers.
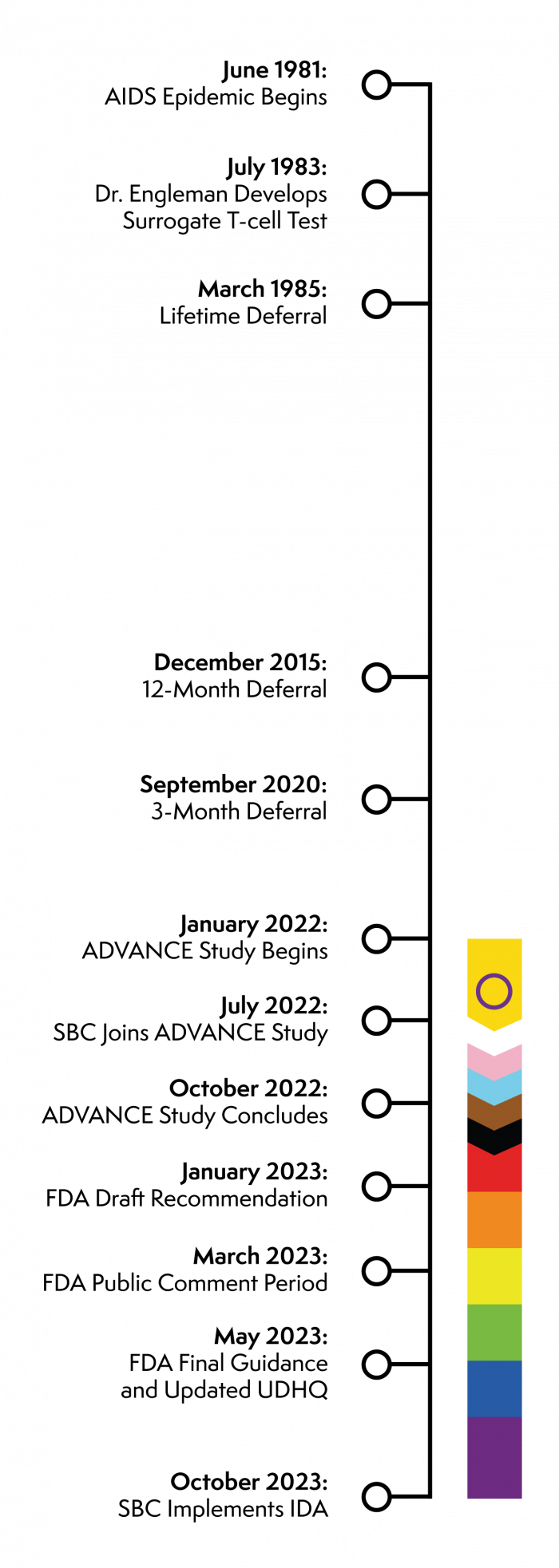 The following is a timeline of the MSM deferral and transition to the Individual Donor Assessment (IDA).
The following is a timeline of the MSM deferral and transition to the Individual Donor Assessment (IDA).
- June 1981: AIDS epidemic is formally recognized by the CDC
- July 1983: Edgar Engleman develops surrogate T-cell testto screen blood supplies for HIV.
- March 1985: Every donor began to be screened for the presence of HTLV-III and assessed if they have a risk factor for AIDS. In September 1985, the permanent deferral of males who have had sex with another man since 1977 was instituted because of the increased risk of HIV infection. During the AIDS epidemic, the technology was nascent at best for both diagnosis and treatment of HIV.
- December 2015: The FDA changed the eligibility criteria of MSM donors from permanently deferred to a 12-month deferral provided the donor had sexual abstinence within the time frame. This was a result of a multi-agency effort to determine if there was scientific evidence (surveillance data) that could guide the change in policy.
- September 2020: The FDA reduced the MSM deferral to three (3) months in alignment with data analysis that supported no increased risk to the blood supply, and changes were escalated in response to a public health emergency and urgent need for blood donors during the Covid-19 pandemic.
- January 2022:The ADVANCE Study launches.
- July 2022:Stanford Blood Center’s Hillview location becomes a study enrollment site. Dr. Suchi Pandey led the project team, comprised of Medical Services, Donor Services, Technical Services, QRA, OpEx, MarCom and others.
- October 2022:The ADVANCE Study concludes.
- January 2023:The FDA issues draft recommendations, proposing a change from time-based deferral to assessing blood donor eligibility using gender-inclusive, individual risk-based questions to reduce the risk of transfusion-transmitted HIV.
- March 2023: The FDA enters its 60-day public comment period.
- May 2023:The FDA finalizes its guidance for the new Individual Donor Assessment; AABB, having worked in concert with the FDA’s timeline, releases the revised Universal Donor History Questionnaire.
- June – October 2023:SBC begins implementation planning, which involves changes to regulatory policies and standard operating procedures, IT systems, marketing materials and communications, as well as extensive training.
- October 2023:Stanford Blood Center fully adopts the Individual Donor Assessment, launching the revised questionnaire.
Yes, since this helps to ensure the safest possible blood supply. All donors must be asked the screening questions at each donation. Both AABB and FDA regulations specifically require that all blood donors complete the donor history questionnaire on the day of donation and prior to donating.
All are welcome to join Stanford Blood Center in supporting local patients, and there are many ways to help. You can host a blood drive at work or with your community organization, volunteer with us, donate blood products for research, or help spread the word! We look forward to having you as part of our family.